Home
The National Ethics Committee was created through the DOST Special Order 84-053 series of 1984, an initiative of the then Executive Director, Dr. Alberto G. Romualdez, Jr. to promote ethics review in health research. Tasked to ensure that all biomedical researches involving human participants conform to international ethical principles and standards towards respect for the sanctity of life and dignity of person, NEC put together the first set of national guidelines for the conduct of biomedical research in 1985. In 2010, the NEC was temporarily phased out (DOST Special Order # 383) only to be reactivated on 9 December 2013 because of the pressing need for a national body to review researches which are of national importance.
On 09 January 2023, the PCHRD issued Special Order no. 23-006, series of 2023, titled Amendment of Functions and Reconstitution of the Membership of the National Ethics Committee (NEC), as follows:
- Ethics review of research protocols that are government-endorsed and are:
- Received through the Philippine Health Research Ethics Portal (PHREP);
- National in scope in terms of study participants; and
- other researches as NEC may deem appropriate for its review.
- Assist institutional research ethics committees in the resolution of difficult ethical issues;
- Provide input to PCHRD and other government agencies including the Food and Drug Administration of the Philippines regarding identified ethical issues in relevant research activities;
- Provide appropriate information to the Philippine Health Research Ethics Board (PHREB) in the formulation of policies and guidelines in health research; and
- Network with other national ethics bodies (e.g., SJREB, PGC-ELSI Program) in contributing to the development of an ethical research environment.
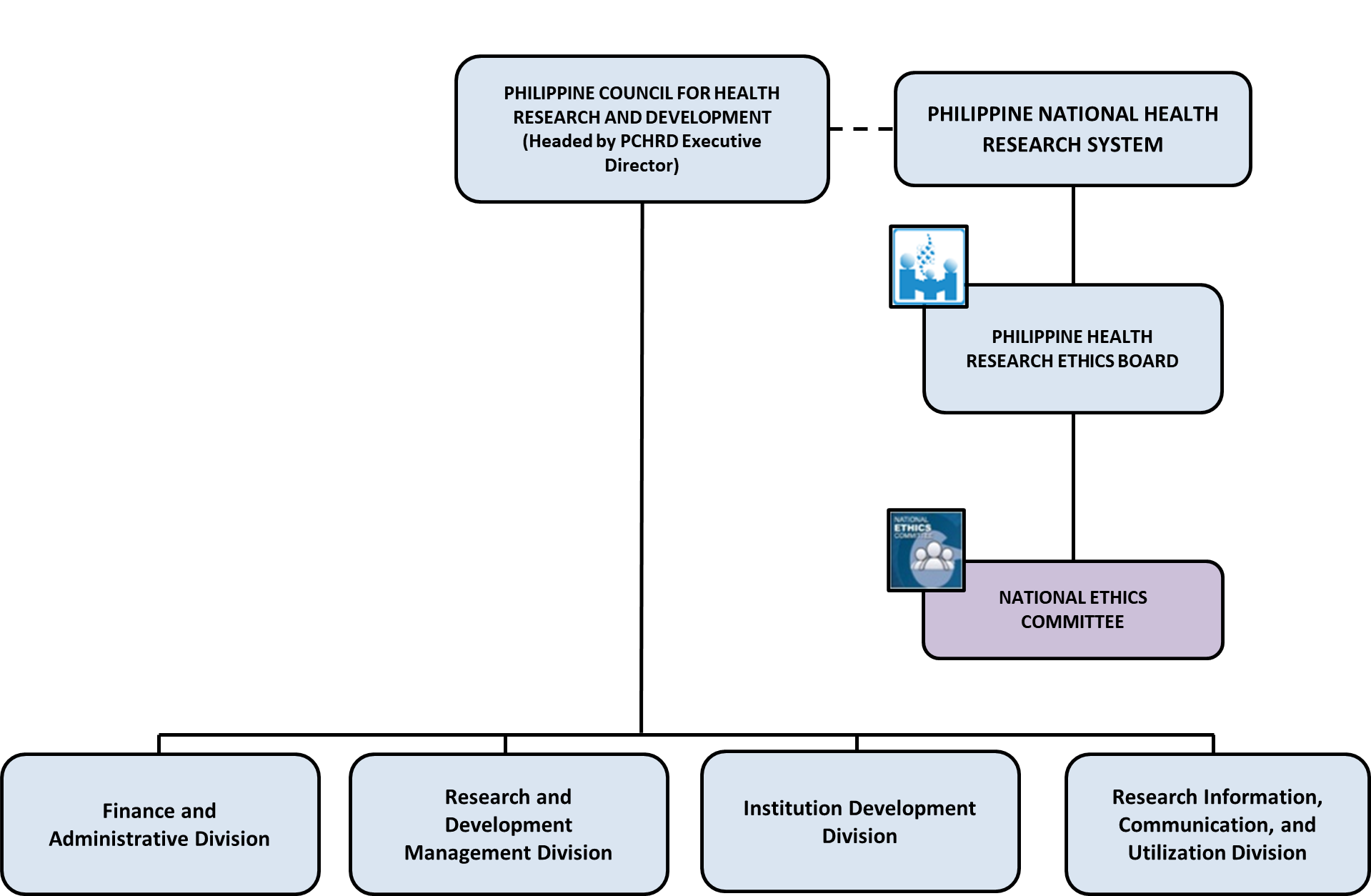
The NEC is envisioned to be an essential component of the Philippine Council for Health Research and Development and the Philippine National Health Research System depicted in the organogram below: